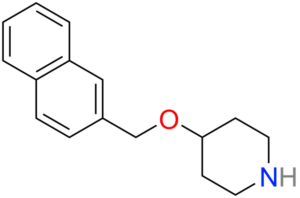
Litoxetine (IXA-001)
Litoxetine (IXA-001) is a selective serotonin (5-HT) reuptake inhibitor (SSRI) and mixed serotonin agonist-antagonist. SSRIs affect the chemicals that nerves in the central nervous system use to send messages to one another.
These chemical messengers, called neurotransmitters, are released by one nerve and taken up by other nerves.
Neurotransmitters that are not taken up by other nerves are taken up by the same nerves that released them. This process is termed « reuptake. » SSRIs work by inhibiting the reuptake of serotonin, an action that allows more serotonin to be available to be taken up by other nerves.
In addition to these effects, Litoxetine has a direct agonist or antagonist effect on specific subtypes of serotonin receptors which makes it particularly appropriate for treating continence dysfunctions.
Scientific data suggests that serotonin is involved in the control of micturition and continence, and this study explores if the specific actions of Litoxetine on the serotoninergic system can improve urinary continence.
Indications: Urinary Incontinence (UI).